Time management is very much important in IIT JAM. The eduncle test series for IIT JAM Mathematical Statistics helped me a lot in this portion. I am very thankful to the test series I bought from eduncle.
Nilanjan Bhowmick AIR 3, CSIR NET (Earth Science)
Debanjana Adhikari posted an Question
- CSIR NET
- Physical Sciences
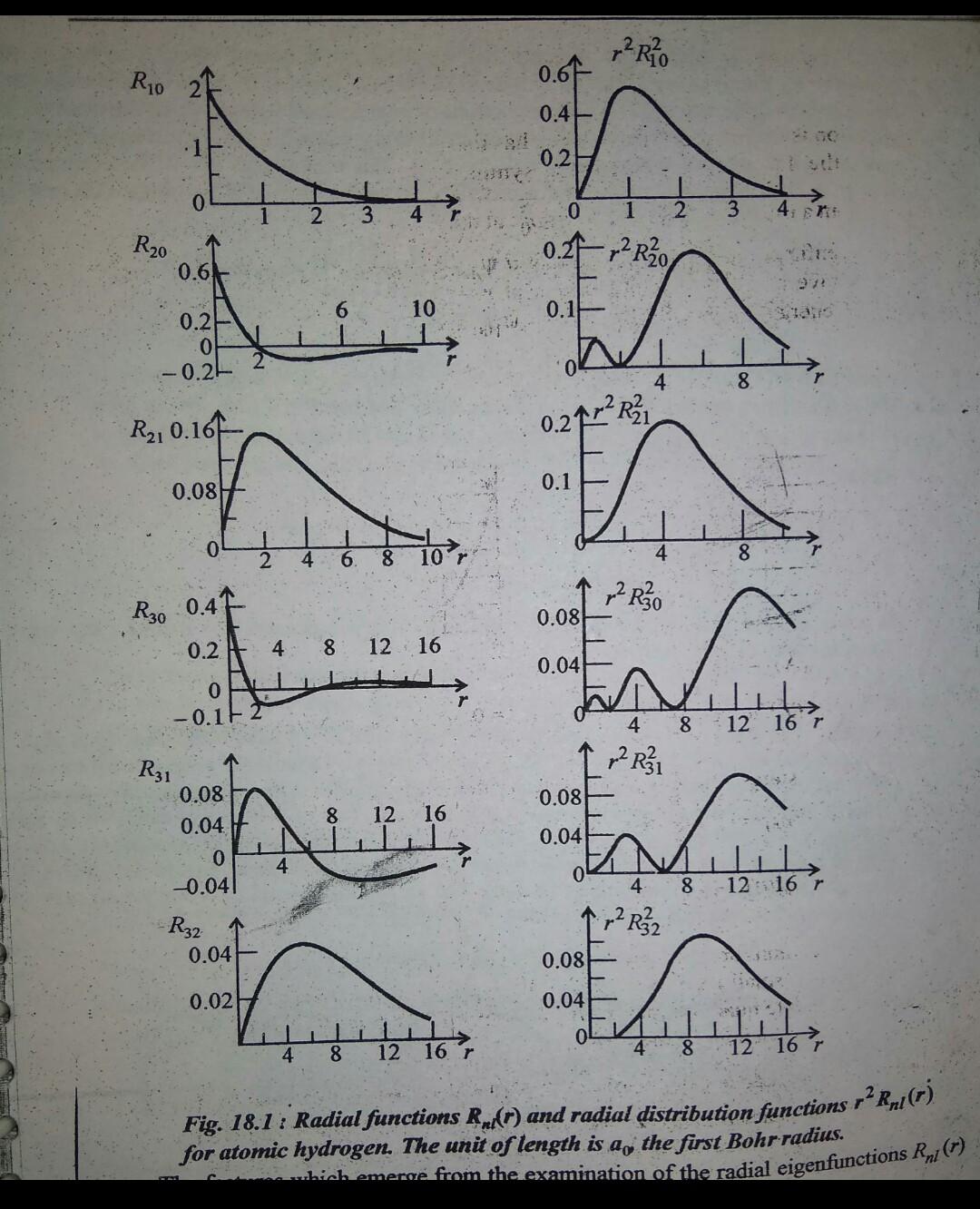
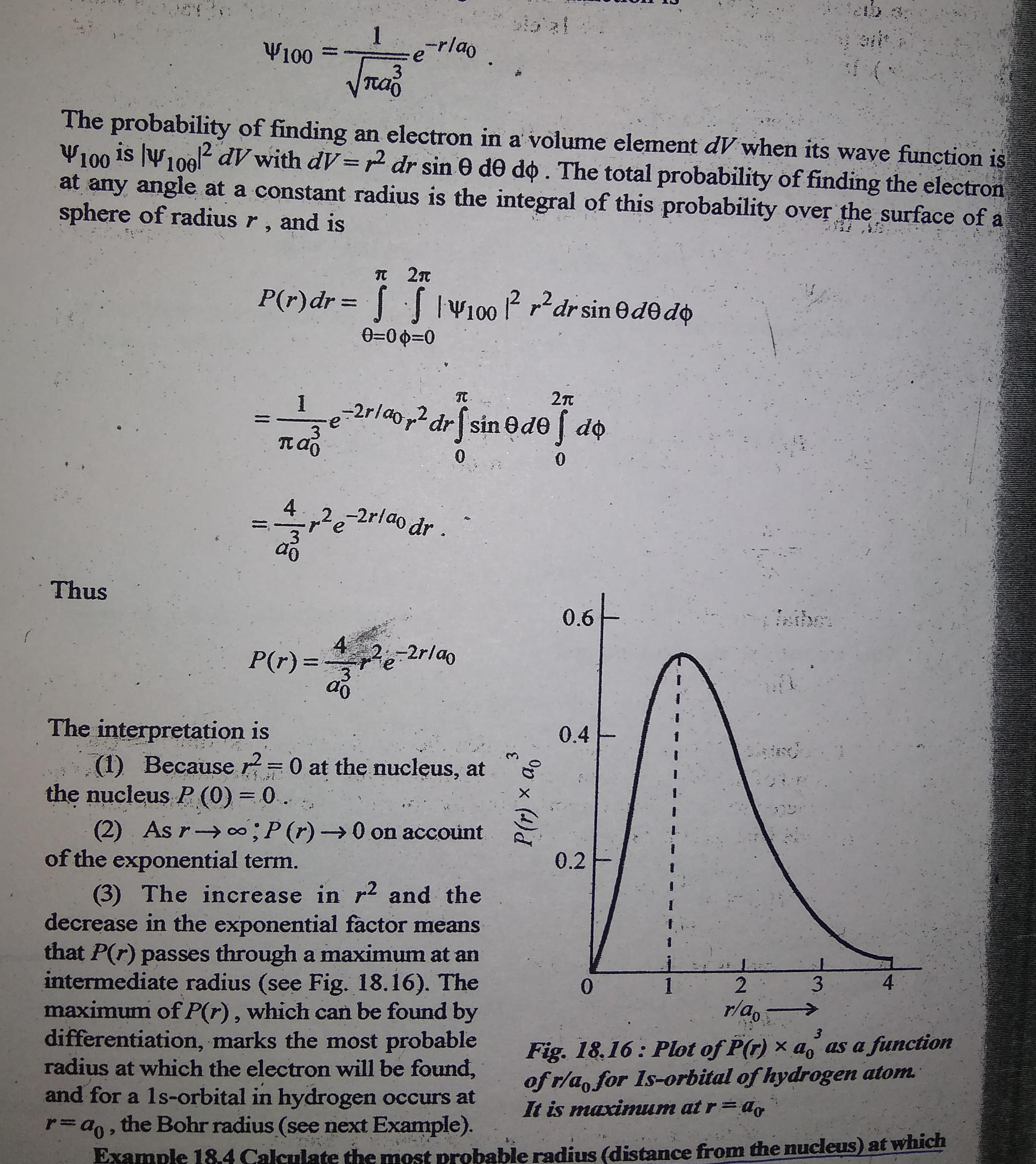
Q18. the ground state wavefunction for the hydrogen atom is given by e, where a, is the bohr radius. the plot of the radial probability density, pr) for the hyd
Q18. The ground state wavefunction for the hydrogen atom Is given by e, where a, is the Bohr radius. The plot of the radial probability density, Pr) for the hydrogen atom in the ground state is (6) (a) Pr PT) (c) (d) P PO I/do I/ao
- 0 Likes
- 5 Comments
- 0 Shares
-
![comment-profile-img]() >
>
-
![comment-profile-img]() >
>
-
![comment-profile-img]() >
>
Dhairya sharma
I already answered see attached
-
![comment-profile-img]() >
>
Do You Want Better RANK in Your Exam?
Start Your Preparations with Eduncle’s FREE Study Material
- Updated Syllabus, Paper Pattern & Full Exam Details
- Sample Theory of Most Important Topic
- Model Test Paper with Detailed Solutions
- Last 5 Years Question Papers & Answers
Sign Up to Download FREE Study Material Worth Rs. 500/-










 >
>
 >
>
 >
>











Chandra prakash
solution