Time management is very much important in IIT JAM. The eduncle test series for IIT JAM Mathematical Statistics helped me a lot in this portion. I am very thankful to the test series I bought from eduncle.
Nilanjan Bhowmick AIR 3, CSIR NET (Earth Science)- IIT JAM
- Chemistry (CY)
How many elements would be in the second period of the periodic table if the spin quantum 1 ,0, or + numbers could have the value,0, or +?
- 0 Likes
- 5 Comments
- 0 Shares
-
![comment-profile-img]() >
>
-
![comment-profile-img]() >
>
-
![comment-profile-img]() >
>
![eduncle-logo-app]()
got it??
![eduncle-logo-app]()
check this simple solution
![eduncle-logo-app]()
generally one orbitals have two electrons which have two spins +1/2 and -1/2 but in this question 1 extra spin is given ie., 0 so total elwctrons in one orbital will be 3
![eduncle-logo-app]()
here 2s and 2p includes total 4 orbitals so total elwctrons will ne 4 ×3 = 12
![eduncle-logo-app]()
got it now?
-
Suman Kumar
![best-answer]()
2s + 2p = 4 orbital = 4*3 = 12 element
Do You Want Better RANK in Your Exam?
Start Your Preparations with Eduncle’s FREE Study Material
- Updated Syllabus, Paper Pattern & Full Exam Details
- Sample Theory of Most Important Topic
- Model Test Paper with Detailed Solutions
- Last 5 Years Question Papers & Answers
Sign Up to Download FREE Study Material Worth Rs. 500/-











 >
>

 >
>


 >
>
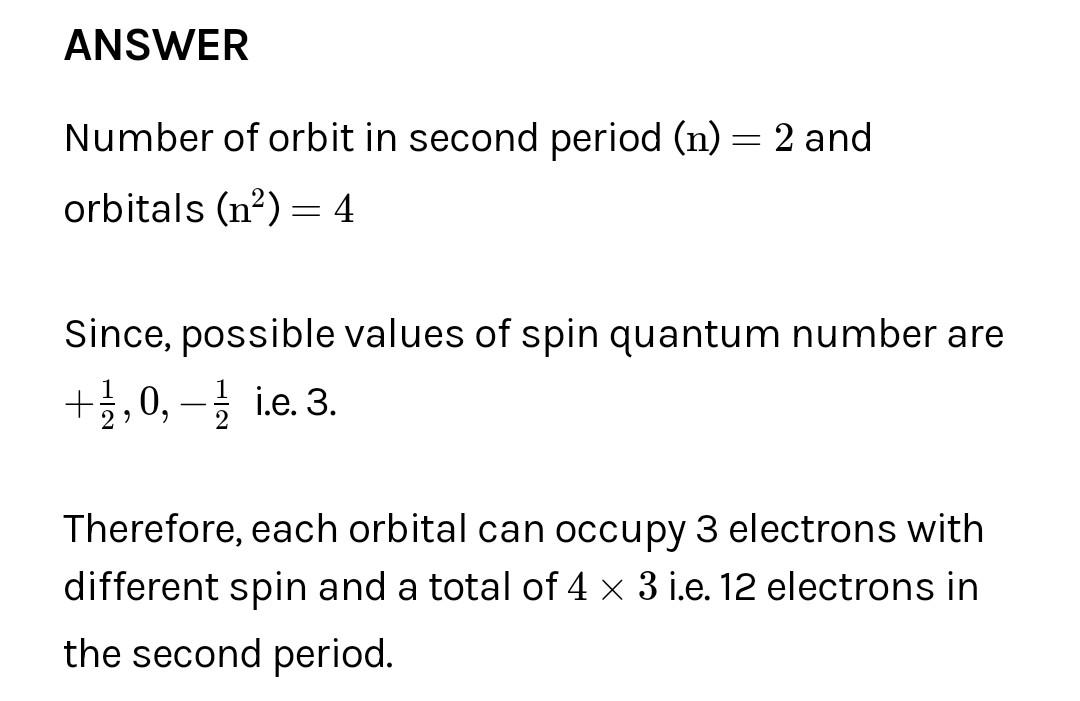







Lingareddy 1
Number of orbit in second period (n) =2 and orbitals (n2) =4 Since, possible values of spin quantum number are +21,0,−21 i.e. 3. Therefore, each orbital can occupy 3 electrons with different spin and a total of 4×3 i.e. 12 electrons in the second period. or n=2 l=0 m =0 s= 1/2, 0, -1/2 l=1 m=0 s= 1/2, 0, -1/2 l=1 m=1 s= 1/2, 0, -1/2 l=1 m= -1 s= 1/2, 0, -1/2 Total 3+3+3+3 =12