The periodic table is considered the very first stage of studying Chemistry. Simply put, it contains all the chemical elements known to mankind according to their similar properties.
In this blog, we are going to discuss all the elements of the periodic table with names, symbols, properties, and easy ways to learn chemistry periodic table with the help of mnemonics and song.
Periodic Classification of Elements
Practice Questions for Periodic Table - Chemistry
Download IIT JAM 2023 Chemistry Syllabus PDF Free
Importance of Periodic Table
There are a total of 118 elements known so far and the discovery of new elements still continues. Every element has its own importance in the environment, some of them are useful while others are harmful. The harmful elements may also be useful in other ways.
It becomes a necessity to study the chemical and physical properties of each of the elements present around us because every element is related to our lives in one way or another. As the number is very large and there is the possibility that more elements will be discovered, so it becomes very difficult to study each of them separately. We need to classify them into groups so that we can study them easily and effectively. The periodic table is the tool that is used to classify the known elements in groups.
It helps us to undertake a systematic study of the various elements found in nature without which it would have been impossible for us to study all the elements in the table. With the help of a periodic table, a comparative study of the elements and their compounds can be done.
Periodic table also helps us to analyze the periodic trend in various properties such as ionization potential, electron affinity, electronegativity etc.
It is a very basic chapter of chemistry It helps us to understand all the chapters of chemistry so we need to learn before starting any chapter of chemistry. Also, it is an important chapter regarding IIT JAM Syllabus as 1 to 2 questions are asked every year.

Periodic Classification of Elements
Classification means identifying similar species and grouping them together. Lavoisier divided elements into two main types known as metals and non-metals.
Dobereiner Law of Triads, Dobereiner grouped the elements in triads (groups of three elements), in such a way that the middle element of the triad had both atomic mass and properties roughly equal to the average of the other two elements of the triad.
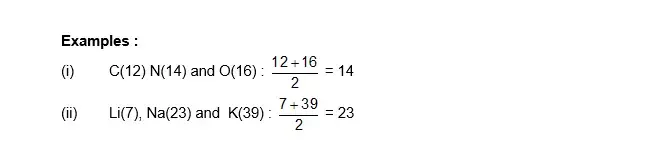
Newland law of Octaves: Newland gave the law of octaves, which states that: 'When elements are arranged in increasing order of their atomic mass, every eighth element beginning from any element resembles the first element in its physical and chemical properties.
This method was also discarded since it failed to accommodate the heavier elements.
Mendeleev's Periodic Table
Mendeleev published a table of elements called Mendeleev’s periodic table. He arranged the elements in the increasing order of their atomic masses. This arrangement enabled Mendeleev to place elements in vertical columns known as groups and in horizontal rows known as periods.
Mendeleev's Periodic Law
The physical and chemical properties of elements are periodic functions of their atomic weights.
| Series |
Group-I |
Group-II |
Group-III |
Group-IV |
Group-V |
Group-VI |
Group-VII |
Group-VIII |
| 1 |
H = 1 |
|||||||
| 2 |
Li = 7 |
Be = 9.4 |
B = 11 |
C = 12 |
N = 14 |
O = 16 |
F = 19 |
|
| 3 |
Na = 23 |
Mg = 24 |
Al = 27.3 |
Si = 28 |
P = 31 |
S = 32 |
Cl = 35.5 |
Mendeleev’s Original Periodic Table of Elements – [First Three Periods shown above]
Download the Eduncle study material for your subjects and start your preparation now.
| Download Free IIT JAM Study Material for All Subjects [Trusted & Recommended by 4600+ Selected Students] |
| Updated Syllabus for All Subjects |
| Sample Theory Notes |
| Model Test Papers with Solution (Section 1, 2 & 3) |
| 5 Previous Exam Papers with Answer Keys |
| Complete Study Plan for all 3 Sections |
| Paper Analysis by Eduncle Experts |
| Personalized Guidance by Subject Experts |
| Get Free Demo Class |
Merits of Mendeleev's Periodic Table:
Elements were arranged in increasing order of atomic weights in horizontal rows called 'periods' and vertical columns called 'groups'.
Elements that are similar with respect to their chemical properties are grouped together and have atomic weights of nearly the same value.
Elements in the same group had the same 'valency' and similar chemical properties.
Based on the periodicity of properties a number of gaps were left in the table for undiscovered elements i.e. elements now discovered e.g. Scandium, Gallium, and Germanium originally called eka-boron, eka-aluminium, and eka-silicon respectively.
The properties of the undiscovered elements left in the vacant gaps were predicted.
Incorrect atomic weights of some of the arranged elements were corrected with the knowledge of the atomic weights of the adjacent elements.
Defects of Mendeleev's Periodic Table:
Certain pairs of elements having higher atomic weights have been given positions before the elements having lower atomic weights.
This defect disappears if elements were arranged according to their atomic numbers. e.g. Co [at. wt. 58.9, at. no. 27) was placed before Ni [at. wt. 58.6, at. no. 28).
The position of rare earth and actinides could be justified only if arranged according to its atomic numbers.
The position of isotopes had to be placed in the same position according to atomic numbers.
Download IIT JAM 2023 Notes & Theory for Free
Modern Periodic Table
The defects of the Mendeleev periodic table were removed by Henry Moseley who put forward the modern periodic law in 1913.
Modern Periodic Law: This law states that the physical and chemical properties of elements are the periodic functions of their atomic number i.e., if the elements are arranged in tabular form in the increasing order of their atomic numbers, then the properties of the elements are repeated after definite regular intervals or periods.
Salient Features of the Modern Periodic Table:
According to modern periodic law, “the properties of elements are the periodic functions of their atomic numbers”.
On the basis of the modern periodic law, a scientist named Bohr proposed a long form of the periodic table that was prepared by Rang and Warner.
In the modern periodic table: the horizontal lines are periods and the vertical lines are groups
The periodic table has a total of 7 periods and 18 groups. But according to the CAS system, the number of groups is 16, because the eighth group has been divided into three groups.
If elements are arranged in increasing order of their atomic numbers, there is a repetition of properties after 2, 8, 18, and 32 elements.
There are two elements in the first period, eight elements in each of the second and third periods, eighteen elements in each of the fourth and fifth periods, thirty-two elements in the sixth period, and only nineteen elements till now in the seventh period.
Table : Modern Periodic
| Periods |
Stable Electronic Configuration |
Number of Electrons |
From |
To |
| First |
1s2 |
2 |
H (1) |
He (2) |
| Second |
2s22p6 |
8 |
Li (3) |
Ne (10) |
| Third |
3s23p6 |
8 |
Na (11) |
Ar (18) |
| Fourth |
4s23d104p6 |
18 |
K (19) |
Kr (36) |
| Fifth |
5s24d105p6 |
18 |
Rb (37) |
Xe (54) |
| Sixth |
6s24f14sd106p6 |
32 (Including lanthanoids) |
Cs (55) |
Rn (86) |
| Seventh |
_ |
19 (Including Actinoids) |
Fr (87) |
Db (105) |
Periodic Classification of Elements
(i) s-block Elements :
The elements of the periodic table in which the last electron enters in s-subshell, are called s-block elements.
The General configuration is ns1–2, where n represents the outermost shell.
The total number of s-block elements is 14.
I A group elements are known as alkali metals whereas the elements of II A group are known as alkaline earth metals.
Fr87 and Ra88 are radio-active elements.
H and He are gaseous elements and Cs and Fr liquid elements.
(ii) p-block Elements :
The elements of the periodic table in which the last electron enters in the p-subshell are called p-block elements.
p-block contains elements groups 13, 14, 15, 16, 17, and 18 of the periodic table.
The general configuration of p-block elements are ns2np1-6 (where n = 2 to 6).
The total number of p-block elements in the periodic table is 30 (excluding He).
Most of these elements are non-metals, some are metalloids and few others are heavy elements that exhibit metallic character.
(iii) d-block Elements :
The elements of the periodic table in which the last electron gets filled up the d-orbital called d-block elements.
The general electronic configuration is (n-1)d1-10, ns1-2.
The d-block contains elements of groups 3 to 12 of the periodic table.
In d-block elements, the electron gets filled up in the d-orbital of the penultimate shell, that is why these elements are known as Transition elements.
The total d-block elements in the periodic table are 33.
Out of all the d-block elements, mercury is the only liquid element.
(iv) f-block Elements :
The elements of the periodic table in which the last electron gets filled up in the f orbital, called f-block elements.
There are 28 f-block elements in the periodic table.
The elements from atomic numbers 58 to 71 are called lanthanides because they come after lanthanum (57).
The elements from atomic numbers from 90 to 103 are called Actinides because they come after actinium (39).
All the actinium elements are radioactive.
The general electronic configuration of these elements is (n-2)f1-14(n-1)d0-1, ns2.
All the elements after atomic number 92 (i.e., U92) are transuranic elements.
Download IIT JAM Previous Papers & Answers for Free
Practice Questions for Periodic Table - Chemistry
Download the Practice Question PDF for the chemistry periodic table and get the solutions for all the below-mentioned questions.
Basic Level Questions on Periodic Table:


We hope these notes helped you to learn the periodic table of elements. If you have any query, then share it with us through the comment box below.
You can also join India’s No.1 Learning Community for IIT JAM, where you will get doubt solutions for all your exam-related queries with the help of experts from all over India. To join the community, you can download the Eduncle App now.
Thank You!
























